Showing 97–108 of 191 results
-
Lonsurf
FreeTrade Name Lonsurf Active Ingredient Triffuridine/Tipiracil Strength 15mg, 20mg Type/form Tablets Manufacturer Taiho Pharm -
Lucentis
FreeTrade Name Lucentis Active Ingredient Ranibizumab Strength 10 mg Type/form Injection Manufacturer Novartis -
Lynparza
FreeMedicine Name Lynparza Generic Name Olaparib Strength Tablets: 100 mg, 150 mg Manufacturer AstraZeneca -
Mabthera
FreeTrade Name Mabthera Active Ingredient Rituximab Strength 100mg, 500mg,1400mg Type/form Injection Manufacturer Roche -
Mabura
FreeTrade Name Mabura Molecule Tocilizumab Strength 80 mg, 200 mg, 400 mg Type/Form Injection (IV/SC) Manufacturer Hetero -
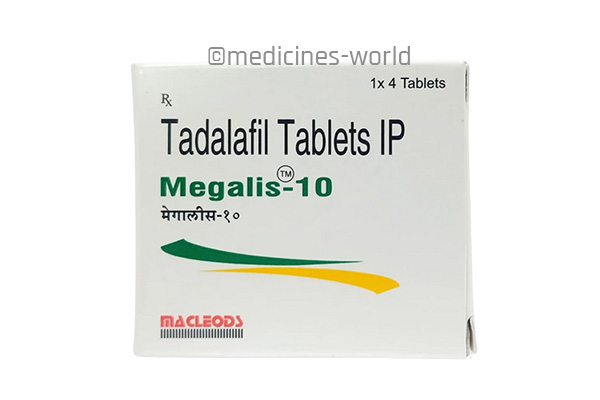
Megalis
FreeTrade Name Megalis 10/20 Molecule Tadalafil Strength 10 mg, 20 mg Type/form Tablet Manufacturer Macleods Pharmaceuticals Pvt. Ltd -
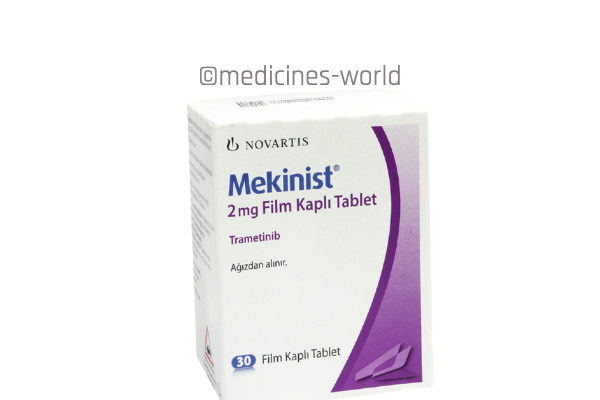
Mekinist
FreeTrade Name Mekinist Active Ingredient Trametinib Strength 0.5mg, 2mg Type/form Tablets Manufacturer Novartis -
Mektovi
FreeTrade Name Mektovi Active Ingredient Binimetinib Strength 15 mg Type/form Tablets Manufacturer Pierre Fabre -
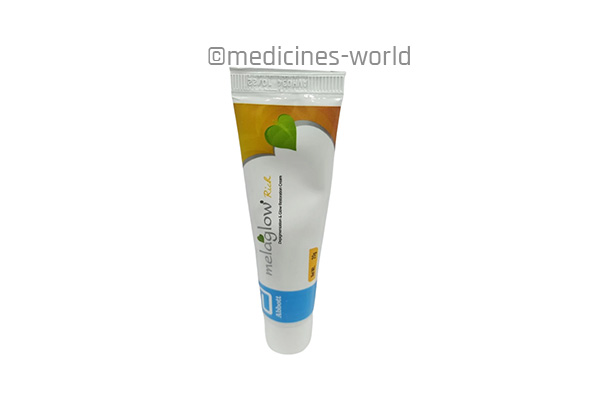
Melaglow Rich
FreeTrade Name Melaglow Rich Molecule Combination of skin-brightening and depigmenting agents Strength Active ingredients include Niacinamide (4%), Arbutin (2%), Licorice Extract (0.12%), and Pterowhite (0.12%) Type / form Cream Manufacturer Abbott Healthcare Pvt. Ltd. -
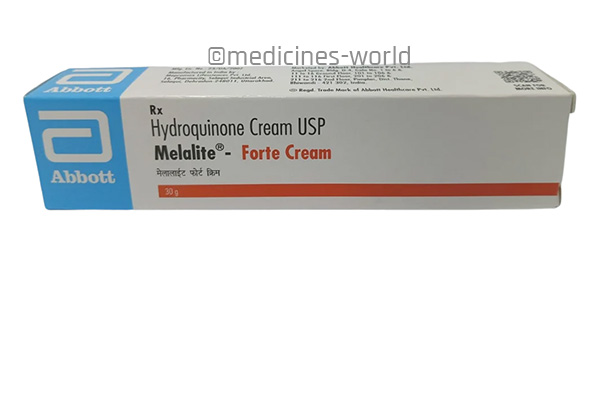
Melalite – Forte Cream
FreeTrade Name Melalite – Forte Cream Molecule Hydroquinone Strength 2% or 4% Type/form Cream Manufacturer Abbott Healthcare Pvt. Ltd. -
MiRel
FreeTrade Name MiRel Molecule Recombinant Tissue Plasminogen Activator Strength 100 mg/4 mL, 400 mg/16 mL Type/Form Injection (IV) Manufacturer Reliance Life Sciences -
Mounjaro
FreeTrade Name Mounjaro Active Ingredient Tirzepatide Strength 2.5mg, 5mg, 7.5mg, 12.5mg Type/form Injection Manufacturer Eli Lily
How It Works
Your Order, From Start to Delivery
Enquire & Get a Quote
Click the WhatsApp link to connect with our team instantly, or drop a note via “Contact Us.” Just share what you need — we’ll get back with the best possible quote, customized for you.
Approve Quote & Complete Payment
Once you're happy with the quote, proceed with the payment to confirm your order. Secure, fast, and hassle-free process with instant confirmation.
Shipment and Processing
We’ll share estimated delivery timelines and begin processing your order. Our logistics team will handle all compliance checks and ensure your shipment is managed with care.
Instant Shipment Status & Updates
Stay informed! Our customer support team will keep you updated regularly with tracking details and delivery status until your medicines are safely delivered to your doorstep.