Showing 13–24 of 191 results
-
Balversa
FreeTrade Name Balversa Active Ingredient Erdafitinib Strength 3mg, 4mg, 5mg Type/form Tablets Manufacturer Janssen -
Basalog One
FreeTrade Name Basalog Molecule Insulin Glargine Strength 100 IU/mL Type/Form Injection (SC) Manufacturer Biocon -
Benlysta
FreeTrade Name Benlysta Active Ingredient Belimumab Strength 400mg Type/form Injection Manufacturer GSK -
Besponsa
FreeTrade Name Besponsa Active Ingredient Inotuzumab Ozogamicin Strength 1 mg/ml Type/form Injection Manufacturer Pfizer -
Betanis®/MyrbetriqTM/Betmiga
FreeTrade Name Betanis®/MyrbetriqTM/Betmiga Molecule Mirabegron Strength 25mg, 50mg Type/Form Extended-release tablet Manufacturer Astellas Pharma -
BevaciRel
FreeTrade Name BevaciRel Molecule Bevacizumab Strength 100 mg/4 mL, 400 mg/16 mL Type/Form Injection (IV) Manufacturer Reliance Life Sciences -
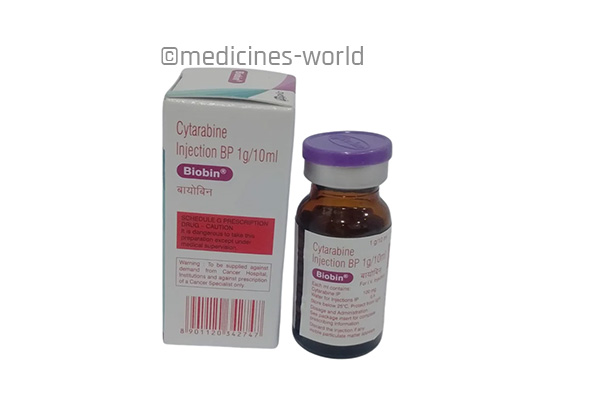
Biobin
FreeTrade Name Biobin Molecule Cytarabine Strength 100 mg, 500 mg, 1000 mg Type/form Injection Manufacturer Zydus Life Sciences -
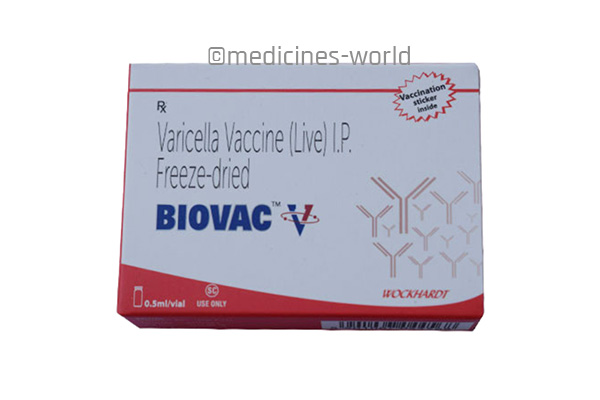
Biovac V
FreeTrade Name Biovac V Molecule Hepatitis B vaccine Strength 10 mcg/0.5 mL, 20 mcg/1 mL Type/Form Injection Manufacturer Wockhardt -
Braftovi
FreeTrade Name Braftovi Active Ingredient Encorafenib Strength 75 mg Type/form Capsules Manufacturer Pierre Fabre -
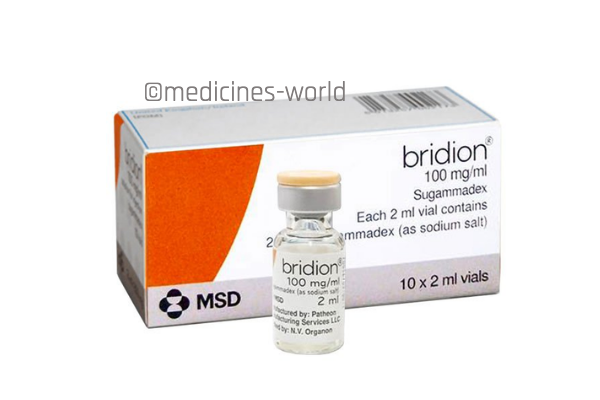
Bridion
FreeTrade Name Bridion Active Ingredient Sugammadex Sodium Strength 100 mg/ml Type/form Injection Manufacturer MSD -
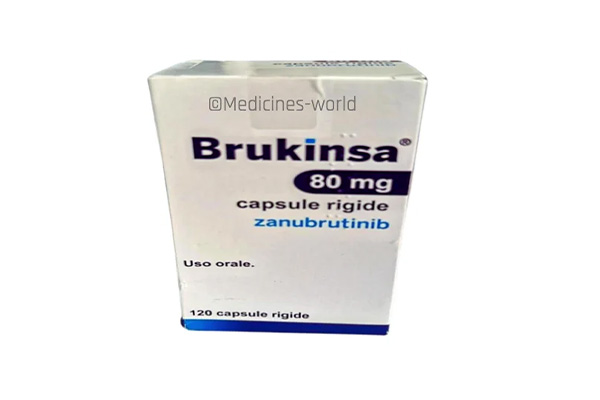
Brukinsa
FreeTrade Name Brukinsa Molecule Zanubrutinib Strength 80mg, 160mg Type/Form Capsule Manufacturer BeiGene -
Cabenuva
FreeTrade Name Cabenuva Active Ingredient Cabotegravir, Rilpivirine Strength 200mg/ml, 300mg Type/form Injection Manufacturer Gsk
How It Works
Your Order, From Start to Delivery
Enquire & Get a Quote
Click the WhatsApp link to connect with our team instantly, or drop a note via “Contact Us.” Just share what you need — we’ll get back with the best possible quote, customized for you.
Approve Quote & Complete Payment
Once you're happy with the quote, proceed with the payment to confirm your order. Secure, fast, and hassle-free process with instant confirmation.
Shipment and Processing
We’ll share estimated delivery timelines and begin processing your order. Our logistics team will handle all compliance checks and ensure your shipment is managed with care.
Instant Shipment Status & Updates
Stay informed! Our customer support team will keep you updated regularly with tracking details and delivery status until your medicines are safely delivered to your doorstep.